Gutanga Uruganda 4-Bromo-2-Acide Fluorobenzoic 4-Bromo-2-Acide Fluorobenzoic CAS 112704-79-7
Gutanga Uruganda 4-Bromo-2-Acide Fluorobenzoic 4-Bromo-2-Acide Fluorobenzoic CAS 112704-79-7
Kugirango twuzuze ibyifuzo byabakiriya byateganijwe, ubu dufite abakozi bacu bakomeye kugirango batange ubufasha bukomeye muri rusange burimo kuzamura, kugurisha kwinshi, igenamigambi, guhanga, kugenzura ubuziranenge bwo hejuru, gupakira, ububiko hamwe nibikoresho byo gutanga uruganda 4-Bromo-2 -Fluorobenzoic Acide 4-Bromo-2-Acide Fluorobenzoic CAS 112704-79-7, Turi inyangamugayo kandi turakinguye.Dutegereje uruzinduko rwawe no gushiraho umubano wizewe kandi wigihe kirekire.
Kugirango twuzuze ibyifuzo byabakiriya byateganijwe, ubu dufite abakozi bacu bakomeye kugirango batange ubufasha rusange bukomeye burimo kuzamura, kugurisha kwinshi, igenamigambi, kurema, kugenzura ubuziranenge bwo hejuru, gupakira, ububiko hamwe nibikoresho byaUbushinwa CAS 112704-79-7 na 112704-79-7, Ibicuruzwa byacu byatsindiye izina ryiza muri buri gihugu gifitanye isano.Kuberako ishyirwaho ryikigo cyacu.twakomeje gutsimbarara kubikorwa byacu byo guhanga udushya hamwe nuburyo bugezweho bwo gucunga uburyo bugezweho, dukurura impano zitari nke muri uru ruganda.Dufata igisubizo cyiza nkimiterere yacu yingenzi.
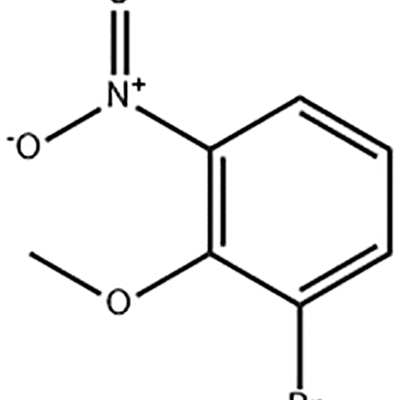
1-Bromo-2-mikorerexy-3-nitro-benzene ikoreshwa nkigihe cya Eltrombopag.
Eltrombopag, yakozwe na GlaxoSmithKline (GSK) mu Bwongereza nyuma iza gukorana na Novartis mu Busuwisi, ni yo ya mbere kandi yemewe ya molekile ntoya itari peptide TPO yakira agonist ku isi.Eltrombopag yemejwe na FDA yo muri Amerika muri 2008 kugirango ivure idiopathic trombocytopenic purpura (ITP), naho muri 2014 yo kuvura anemia ikabije (AA).Ninumuti wambere wemejwe na FDA yo muri Amerika kuvura AA mumyaka 30 ishize.
Ukuboza2012, FDA yo muri Amerika yemeye Eltrombopag kuvura trombocytopenia ku barwayi barwaye hepatite C idakira (CHC), ku buryo abarwayi ba hepatite C bafite uburwayi buke buterwa na platine nkeya bashobora gutangira kandi bagakomeza kuvura bisanzwe bishingiye ku ndwara z’umwijima.Ku ya 3 Gashyantare 2014, GlaxoSmithKline yatangaje ko FDA yatanze impamyabumenyi y’ubuvuzi ya Eltrombopag yo kuvura indwara ya hémopenia ku barwayi bafite imiti ikaze y’imiti idakira (SAA) ititabira byimazeyo ubudahangarwa bw'umubiri.Ku ya 24 Kanama 2015, FDA yo muri Amerika yemeje Eltrombopag kuvura trombocytopenia ku bantu bakuru ndetse n’abana bafite imyaka 1 n’imyaka irenga hamwe na immunite trombocytopenia idakira (ITP) badafite ibisubizo bidahagije kuri corticosteroide, immunoglobuline cyangwa splenectomy.Ku ya 4 Mutarama 2018, Eltrombopag yemerewe gushyirwa ku rutonde mu Bushinwa kugira ngo ivurwe mbere na mbere na trombocytopenia (ITP).
Kugirango twuzuze ibyifuzo byabakiriya byateganijwe, ubu dufite abakozi bacu bakomeye kugirango batange ubufasha bukomeye muri rusange burimo kuzamura, kugurisha kwinshi, igenamigambi, guhanga, kugenzura ubuziranenge bwo hejuru, gupakira, ububiko hamwe nibikoresho byo gutanga uruganda 4-Bromo-2 -Fluorobenzoic Acide 4-Bromo-2-Acide Fluorobenzoic CAS 112704-79-7, Turi inyangamugayo kandi turakinguye.Dutegereje uruzinduko rwawe no gushiraho umubano wizewe kandi wigihe kirekire.
Gutanga UrugandaUbushinwa CAS 112704-79-7 na 112704-79-7, Ibicuruzwa byacu byatsindiye izina ryiza muri buri gihugu gifitanye isano.Kuberako ishyirwaho ryikigo cyacu.twakomeje gutsimbarara kubikorwa byacu byo guhanga udushya hamwe nuburyo bugezweho bwo gucunga uburyo bugezweho, dukurura impano zitari nke muri uru ruganda.Dufata igisubizo cyiza nkimiterere yacu yingenzi.