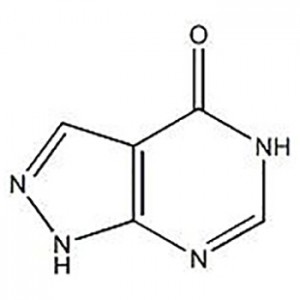
3′-Amino-2′-hydroxy- [1,1'-bipheny] -3-aside aside
3′-Amino-2′-hydroxy- [1,1'-bipheny] -3-aside aside
3'-Amino-2'-hydroxy- [1,1'-bipheny] -3-acide karubike ikoreshwa nka interineti ya Eltrombopag.
Eltrombopag, yakozwe na GlaxoSmithKline (GSK) mu Bwongereza nyuma iza gukorana na Novartis mu Busuwisi, ni yo ya mbere kandi yemewe ya molekile ntoya itari peptide TPO yakira agonist ku isi.Eltrombopag yemejwe na FDA yo muri Amerika muri 2008 kugirango ivure idiopathic trombocytopenic purpura (ITP), naho muri 2014 yo kuvura anemia ikabije (AA).Ninumuti wambere wemejwe na FDA yo muri Amerika kuvura AA mumyaka 30 ishize.
Ukuboza2012, FDA yo muri Amerika yemeye Eltrombopag kuvura trombocytopenia ku barwayi barwaye hepatite C idakira (CHC), ku buryo abarwayi ba hepatite C bafite uburwayi buke buterwa na platine nkeya bashobora gutangira kandi bagakomeza kuvura bisanzwe bishingiye ku ndwara z’umwijima.Ku ya 3 Gashyantare 2014, GlaxoSmithKline yatangaje ko FDA yatanze impamyabumenyi y’ubuvuzi ya Eltrombopag yo kuvura indwara ya hémopenia ku barwayi bafite imiti ikaze y’imiti idakira (SAA) ititabira byimazeyo ubudahangarwa bw'umubiri.Ku ya 24 Kanama 2015, FDA yo muri Amerika yemeje Eltrombopag kuvura trombocytopenia ku bantu bakuru ndetse n’abana bafite imyaka 1 n’imyaka irenga hamwe na immunite trombocytopenia idakira (ITP) badafite ibisubizo bidahagije kuri corticosteroide, immunoglobuline cyangwa splenectomy.Ku ya 4 Mutarama 2018, Eltrombopag yemerewe gushyirwa ku rutonde mu Bushinwa kugira ngo ivurwe mbere na mbere na trombocytopenia (ITP).


![3′-Amino-2′-hydroxy- [1,1'-bipheny] -3-aside aside ya karubike](http://cdn.globalso.com/jindunchem-med/image351.png)
![3′-Amino-2′-hydroxy- [1,1'-bipheny] -3-aside aside](http://cdn.globalso.com/jindunchem-med/image351-300x300.png)