2019 Uburyo bushya 3-Aminopyrazine-2-Acide Carboxylic CAS No 5424-01-1
2019 Uburyo bushya 3-Aminopyrazine-2-Acide Carboxylic CAS No 5424-01-1
Imishinga myinshi yubuyobozi inararibonye hamwe nimwe gusa kumurongo umwe utanga isoko itanga akamaro gakomeye mumatumanaho yumuryango no kumva byoroshye ibyo witeze kuri 2019 Imiterere mishya 3-Aminopyrazine-2-Acide Carboxylic Acide CAS No 5424-01-1, We bafite ubushake bwo kuguha igiciro cyo hasi kumasoko, ubuziranenge bwiza na serivise nziza yo kugurisha.Murakaza neza gukora bussines natwe, reka dutsinde kabiri.
Mubyukuri imishinga myinshi yubuyobozi inararibonye hamwe nimwe gusa kumurongo umwe utanga isoko itanga akamaro gakomeye ko gutumanaho mumashyirahamwe no kumva byoroshye ibyo witezehoUbushinwa 3-Aminopyrazine-2-Acide Carboxylic na 5424-01-1, Ibicuruzwa byacu birazwi cyane mwijambo, nka Amerika yepfo, Afrika, Aziya nibindi.Amasosiyete "gukora ibicuruzwa byo mucyiciro cya mbere" nkintego, kandi yihatire guha abakiriya ibisubizo byujuje ubuziranenge, gutanga serivisi nziza nyuma yo kugurisha no kugoboka tekinike, hamwe ninyungu zabakiriya, bihanga umwuga mwiza nigihe kizaza!
2′-Methoxy-3′-nitro-biphenyl-3-karubasi ya acide ikoreshwa nka interineti ya Eltrombopag.
Eltrombopag, yakozwe na GlaxoSmithKline (GSK) mu Bwongereza nyuma iza gukorana na Novartis mu Busuwisi, ni yo ya mbere kandi yemewe ya molekile ntoya itari peptide TPO yakira agonist ku isi.Eltrombopag yemejwe na FDA yo muri Amerika muri 2008 kugirango ivure idiopathic trombocytopenic purpura (ITP), naho muri 2014 yo kuvura anemia ikabije (AA).Ninumuti wambere wemejwe na FDA yo muri Amerika kuvura AA mumyaka 30 ishize.
Ukuboza2012, FDA yo muri Amerika yemeye Eltrombopag kuvura trombocytopenia ku barwayi barwaye hepatite C idakira (CHC), ku buryo abarwayi ba hepatite C bafite uburwayi buke buterwa na platine nkeya bashobora gutangira kandi bagakomeza kuvura bisanzwe bishingiye ku ndwara z’umwijima.Ku ya 3 Gashyantare 2014, GlaxoSmithKline yatangaje ko FDA yatanze impamyabumenyi y’ubuvuzi ya Eltrombopag yo kuvura indwara ya hémopenia ku barwayi bafite imiti ikaze y’imiti idakira (SAA) ititabira byimazeyo ubudahangarwa bw'umubiri.Ku ya 24 Kanama 2015, FDA yo muri Amerika yemeje Eltrombopag kuvura trombocytopenia ku bantu bakuru ndetse n’abana bafite imyaka 1 n’imyaka irenga hamwe na immunite trombocytopenia idakira (ITP) badafite ibisubizo bidahagije kuri corticosteroide, immunoglobuline cyangwa splenectomy.Ku ya 4 Mutarama 2018, Eltrombopag yemerewe gushyirwa ku rutonde mu Bushinwa kugira ngo ivurwe mbere na mbere na trombocytopenia (ITP).
Imishinga myinshi yubuyobozi inararibonye hamwe nimwe gusa kumurongo umwe utanga isoko itanga akamaro gakomeye mumatumanaho yumuryango no kumva byoroshye ibyo witeze kuri 2019 Imiterere mishya 3-Aminopyrazine-2-Acide Carboxylic Acide CAS No 5424-01-1, We bafite ubushake bwo kuguha igiciro cyo hasi kumasoko, ubuziranenge bwiza na serivise nziza yo kugurisha.Murakaza neza gukora bussines natwe, reka dutsinde kabiri.
2019 Uburyo bushyaUbushinwa 3-Aminopyrazine-2-Acide Carboxylic na 5424-01-1, Ibicuruzwa byacu birazwi cyane mwijambo, nka Amerika yepfo, Afrika, Aziya nibindi.Amasosiyete "gukora ibicuruzwa byo mucyiciro cya mbere" nkintego, kandi yihatire guha abakiriya ibisubizo byujuje ubuziranenge, gutanga serivisi nziza nyuma yo kugurisha no kugoboka tekinike, hamwe ninyungu zabakiriya, bihanga umwuga mwiza nigihe kizaza!



![Igiciro cyo Kurushanwa Kuruganda Rwinshi Hagati ya BMK Pmk Ifu 1-Propanol, 2-Methyl-2- [(phenylmethylene) Amino] - CAS 22563-90-2](http://cdn.globalso.com/jindunchem-med/image351-300x300.png)
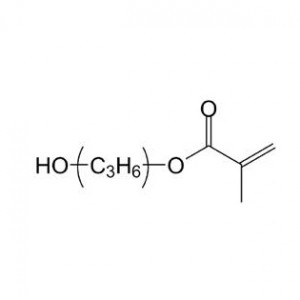
![Ubushinwa bukora 1-Tert-Butoxycarbonyl-4- [(4-fluorophenyl) Amino] Piperidine](http://cdn.globalso.com/jindunchem-med/image111-300x300.png)